
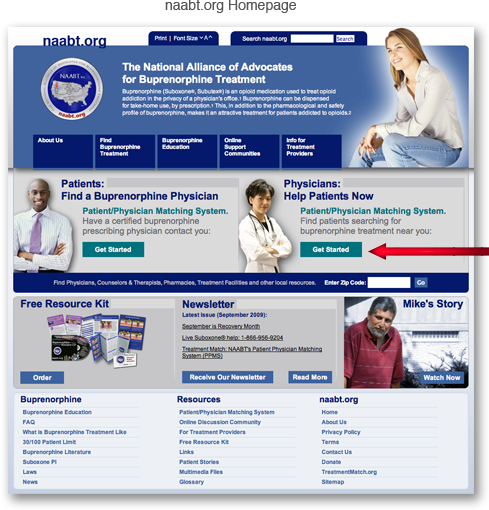
The National Alliance of Advocates
for Buprenorphine Treatment
Buprenorphine (Suboxone®, Subutex®3, Zubsolv®4, Bunavail™5, Probuphine®6) is an opioid medication used to treat opioid addiction in the privacy of a physician's office.1 Buprenorphine can be dispensed for take-home use, by prescription.1 This, in addition to the pharmacological and safety profile of buprenorphine, makes it an attractive treatment for patients addicted to opioids.2
TreatmentMatch.org - Patient/Physician Matching System: What is it?
The free-of-charge matching system is a confidential way for physicians in an office-based practice to find, screen and contact patients seeking buprenorphine treatment for opioid dependence.
How do I get started?
Complete the one-time confidential registration. |
Register |
| Already registered? Click here to login now. | |
 |
Then what?
|
Common Concerns
|
|
For More Information:
The Purpose of Buprenorphine Treatment:
To suppress the debilitating symptoms of cravings and withdrawal, enabling the patient to engage in therapy, counseling and support, so they can implement positive long-term changes in their lives which develops into the new healthy patterns of behavior necessary to achieve sustained addiction remission. - explain -
The National Alliance of Advocates for Buprenorphine Treatment is a non-profit organization charged with the mission to:
- Educate the public about the disease of opioid addiction and the buprenorphine treatment option.
- Help reduce the stigma and discrimination associated with patients with addiction disorders.
- Serve as a conduit connecting patients in need of treatment to buprenorphine treatment providers.
- U.S. Food and Drug Administration, FDA Talk Paper, T0238, October 8, 2002, Subutex and Suboxone approved to treat opiate dependence.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, Md: Substance Abuse and Mental Health Services Administration, 2004.
- Subutex Discontinued in the US market in late 2011.
- Zubsolv (bup/nx sublingual tablet) FDA approved 7/3/2013 see buprenorphine pipeline graphic -in pharmacies now.
- Bunavail (bup/nx bucal film) FDA approved 6/6/2014 see buprenorphine pipeline graphic -in pharmacies now.
- Probuphine FDA approved 5/26/2016 - FDA Probuphine press release
